BACKGROUND
FDA proposes additions to the HPHC list
Nicotine

Aug 9, 2019 | Published by Joanna Marshall
Nicotine
The US Food and Drug Administration (FDA) have recently proposed additions to the constituents or chemicals of concern included on their Harmful and Potentially Harmful Constituent (HPHC) list for tobacco products. This guide outlines the toxicants they propose should appear on the list, the reasoning for the potential additions, and how these changes could impact on companies in the Electronic Nicotine Delivery Systems (ENDS) sector.
Why are FDA adding to the HPHC list?
The current established HPHC list contains 93 constituents that are linked to the five most serious health effects of tobacco use: cancer, cardiovascular disease, respiratory effects, developmental or reproductive effects, and addiction. Under US law, tobacco product manufacturers have been required to report to the FDA levels of HPHCs found in their tobacco products and tobacco smoke since 2009. From May 2016 onwards this rule has also extended to newly-regulated tobacco products, including ENDS products.
The current list of HPHCs was determined prior to the rule extending to ENDS products. Therefore, FDA are considering revising the list so that it reflects the current range of tobacco products i.e. to make it more applicable to ENDS products.
What toxicants do they propose adding?
The list of chemicals FDA propose adding to the HPHC list, applicable to all tobacco products, are as follows:
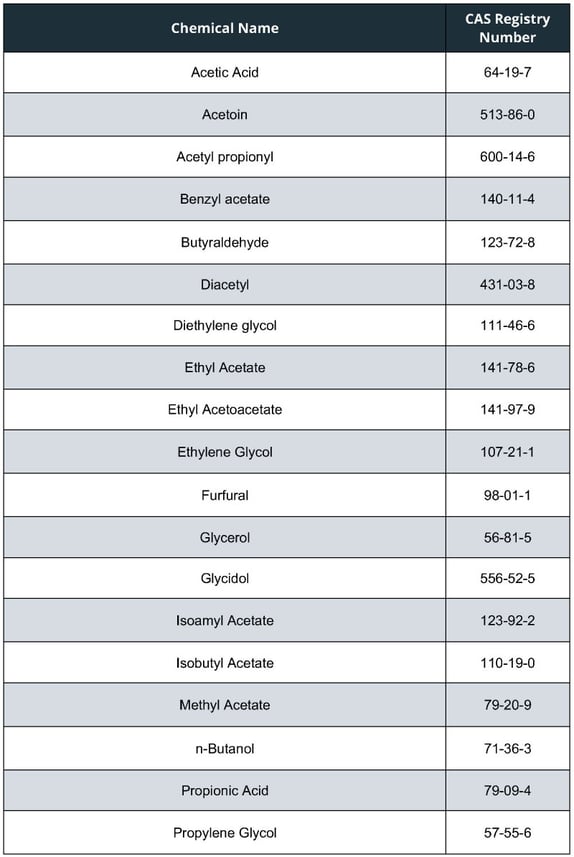
Two of the above (Glycidol and Ethylene Glycol) have been added because they apply under the FDA’s current criterion used to determine whether a constituent should be included on the list. A specific constituent (Diethylene Glycol) appears on the proposed list as the acute health consequences from exposure to products contaminated with this toxicant, which has previously been detected in some e-liquids and ENDS aerosol, can be serious and irreversible.
The remainder of the 19 toxicants which may be added to the list, have been identified due to FDA proposing that one additional criterion be considered when determining whether a constituent should be included on the list – this being chemicals identified by the National Institute for Occupational Safety and Health (NIOSH) as having adverse respiratory effects.
How does this proposed list differ from the new PMTA list?
Additional constituents that FDA have identified as applicable to ENDS products specifically, within their final guidance for Premarket Tobacco Product Applications (PMTAs). This recent proposed change to the HPHC list will potentially be a legal ruling, rather than guidance. All of the chemicals added within the recent PMTA final guidance, appear on the most recent list of proposed additions to the HPHC list or on the current established list.
What does this mean for ENDS companies?
Firstly, once proposed additions to the list are finalized, this will provide increased certainty regarding the HPHCs to incorporate within a PMTA. However, it may be a while until this increased certainty is provided, as FDA are requesting comment on the proposed additions to the list by October 4th, 2019. Therefore, with the PMTA deadline being May 12th 2020, we suggest retaining focus on the HPHCs detailed in the recent final guidance.
Meanwhile, this recent development may suggest that FDA will be preparing a draft guidance and, subsequently, final HPHC reporting guidance in the near future. The date of the final HPHC reporting guidance will determine the HPHC reporting deadline – as earlier this year FDA revised the HPHC reporting date from November 8, 2019 to (6) months from the publication date of a final guidance regarding HPHC reporting under section 904(a)(3)18, or nine (9) months from the publication date of a final guidance regarding HPHCs, for small tobacco product manufacturers. Their recent announcement states that “The FDA intends to provide additional guidance in the future to assist manufacturers or importers of newly-regulated products with compliance with this requirement”. So, watch this space..
A coordinated approach to PMTA and HPHC compliance
Broughton offer an integrated approach to examining the toxicology of HPHCs and flavorants, undertaking clinical consumer use evaluations and implementing efficient analytical testing strategies. To discuss how to ensure your ENDS business meets the forthcoming PMTA & HPHC compliance deadlines, contact us to arrange a meeting.


