Broughton Principal Scientist, Paul Barr, has written a white paper outlining the current...


5 minute read
E-cigarettes have the potential to achieve tobacco harm reduction as part of a controlled off-ramping journey – but there are roadblocks along the way.
Filter
Filter

Broughton Principal Scientist, Paul Barr, has written a white paper outlining the current...

Broughton's Regulatory Operations Manager, Emily Saunders, explains what cold-pressed CBD products...
_MEDICAL.jpg)
Broughton’s Chief Executive Officer, Chris Allen speaks to leading industry publication Cannabis...

Broughton's Regulatory Operations Manager, Emily Saunders discusses the legalization of...

3 minute read
Broughton's Regulatory Operations Manager, Emily Saunders discusses the key themes from Cannabis...
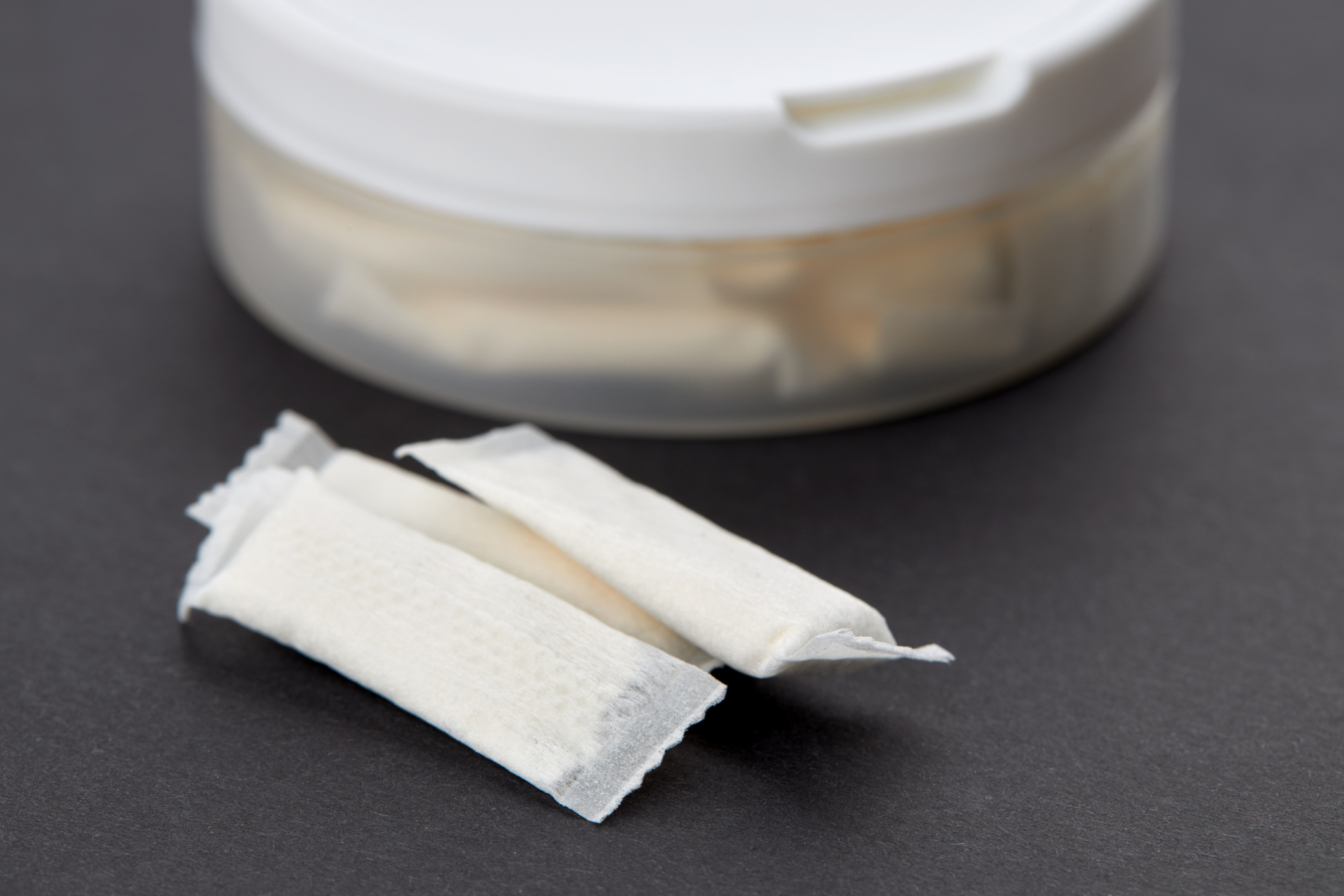
Modern Oral Nicotine Products (MONPs) are another exciting product innovation that can support the...

4 minute read
Broughton's Regulatory Operations Manager, Emily Saunders discusses why lengthy delays in the CBD...

Broughton's Chief Executive Officer, Chris Allen summarizes the highlights from Global Forum on...

Broughton's Chief Commercial Officer, Andy Mooney summarizes the highlights from Next Generation...
Ensure your business stays ahead of the competition by keeping up to date on the latest regulatory changes. Sign up for our newsletter to have the latest information and insights served directly to your inbox.