Last week’s announcement from FDA regarding the authorization of on! PLUS was very encouraging.

Staying on top of regulatory changes, new technical innovations, and industry-disrupting competitors has never been harder. With so much information available, how do you ensure you have the insight you need to drive your business forward and seize opportunities before the competition?
Our regulatory and scientific experts are here to help you stay up to speed with new trends and research. Let them share their expertise and insight so you can focus on building your business.

Browse our resource library to get more insight into the latest industry trends and a better understanding of the right regulatory framework to choose for your innovation or new product. Learn how our scientific and regulatory experts can help create competitive advantage and new business opportunities through their insight and expertise.
Filter

Discover how Broughton’s scientific and regulatory experts help cosmetic brands ensure product safety, validate claims, and achieve global compliance from formulation to market launch.
Click to download
Stay informed with Broughton’s October 2025 Regulatory Update, covering key developments in FDA, MHRA, and EU guidance for nicotine and cannabis products.
Click to download.png?width=776&height=720&name=Nicotine%20Products%20Trends%20(Clear).png)
This paper explores the next wave of alternative nicotine formats beyond traditional pouches and vapes. It examines emerging categories such as oral strips, sprays, hybrids, synthetic nicotine, and inhaler-style devices, and highlights the regulatory, testing, and enforcement measures needed to ensure these products deliver genuine harm reduction while protecting public health.
Click to download
This midyear update from former FDA CTP leader Lillian Ortega explores major 2025 regulatory shifts, PMTA decisions, and what they mean for reduced‑risk products.
Click to download
The United States stands at the forefront of the oral nicotine market, delivering significant commercial opportunities and a well-established PMTA regulatory framework that empowers pouch manufacturers to achieve scalable growth.
Click to download
This checklist helps vape manufacturers and retailers prepare for the UK single-use vape ban. It outlines key steps including product portfolio audits, MHRA compliance, emissions testing, retail withdrawal planning, and environmental readiness.
Click to download
Explore the benefits of CBGA testing for regulatory compliance, product safety, and innovation. Broughton delivers precision analytics for cannabis brands.
Click to download
New UAE regulations for tobacco-free nicotine pouches take effect by June 2025. Broughton helps brands meet compliance with expert-led testing, toxicological risk assessments, and packaging reviews. With a seven-day turnaround and submission-ready reports, our end-to-end support ensures faster, accurate market access with reduced risk of regulatory delays.
Click to download
Use our checklist to get prepared for UAE nicotine pouch regulation changes.
Click to download
Bringing medicinal cannabis products to market can be complex, but your testing doesn’t have to be. Broughton offers a full suite of in-house testing services, including in-process testing, GMP-certified import testing, QC batch release, and ICH-compliant stability studies.
Click to download
Ensure product quality, safety & compliance with Broughton’s ICH-compliant cannabis stability testing. Get precise shelf-life data & real-time monitoring
Click to download
Broughton’s GMP QC Batch Release Testing ensures medicinal cannabis products comply with UK regulations, verifying safety, potency, and label accuracy. Our GMP-certified laboratory streamlines batch approval, reducing delays and ensuring a consistent supply of high-quality, compliant products to the market.
Click to download
Ensure regulatory compliance with Broughton's GMP-Certified Imports Testing for medicinal cannabis. Verify quality, safety & label accuracy for UK market entry.
Click to download
Introducing Broughton’s In-Process Testing Service: a streamlined solution for medicinal cannabis cultivators seeking rapid and efficient cannabinoid content analysis
Click to download
Streamlining the PMTA process for oral nicotine products with tailored strategies and efficient project management.
Click to download
Stability studies are essential in ensuring drug products remain safe, effective, and high-quality throughout their shelf life. While bundled services often include stability testing, hidden costs and limitations can create challenges.
Click to download
Accelerate your nicotine pouches to market with Broughton's comprehensive full-service solutions. This guide details our nicotine pouch testing and additional toxicology packages.
Click to download
Are you a category innovator developing heated tobacco products (HTPs) to help achieve a smoke-free future? Read our expert advice on navigating the regulatory landscape to bring a heated tobacco product to market.
Click to download
As the beneficial effects of medicinal cannabis gain greater acceptance and more consumers begin to use cannabis-derived products, there is an ever-increasing need for cultivators to ensure that their products are tested to the required standards to ensure both product safety and quality.
Click to download
Broughton is a leading global contract research organisation specialising in tobacco harm reduction, medicinal cannabis, cannabinoids, and pharmaceuticals, we can help bring category innovators life-enhancing products to market.
Click to download
New product research requires deep scientific understanding to ensure these products are regulatory compliant and accepted by consumers. Broughton are experts in bringing NGPs to market.
Click to download
As the medical cannabis market grows, expertise in testing, regulatory compliance, and quality control play a pivotal role in assisting category innovators in bringing their products to market.
Click to download
With over 60,000 liters of in-house ICH stability storage capacity, supported by a team of highly qualified scientists and GMP-accredited laboratory testing facilities, we have approximately 250 live studies in progress and offer a full range of stability services to help bring your product to market or ensure its ongoing compliance requirements.
Click to download

Discover how Broughton leads in supporting HTP manufacturers for tobacco harm reduction.
Click to download
Enhancing the positive reputation of cannabis by accelerating safe, efficacious, and high-quality cannabis products to a regulated market. Learn more about our cannabis services.
Click to download
This guide examines the key steps in the cannabis analytical testing process and what cultivators need to consider when selecting a testing partner.
Click to download
At Broughton, our consultants have deep industry knowledge across all the elements required for a PMTA. We guide you through and generate the required data, to ensure that FDA finds your applications strong and compelling.
Click to download

An overview of the Tobacco Products Directive and Tobacco-Related Products Regulations. Get the straightforward answers you’re looking for when it comes to TPD and TRPR analysis.
Click to download
Looking to take your ENDS products to new markets? Compare the differences and get the pros and cons between products with EU Tobacco Products Directive (TPD) notification and products with an EU medicinal product license in our free download.
Click to download
For category innovators thorough stability studies are vital for successful drug development and regulatory approval.
Click to download
We help companies of all sizes through the product lifecycle stages, from concept innovation, product realization, and regulatory submission to post marketing surveillance.
Click to download
The MHRA is now a stand-alone body issuing national authorisations only. This infographic outlines the routes to a Pharmaceutical Marketing Authorisation in the UK.
Click to download
The evaluation of extractables and leachables (E&L) is an essential component of the pharmaceutical development process.
Click to download
Our team of in-house toxicologists reviews and evaluates toxicological data on a wide range of chemicals to produce independent expert opinions on risk assessment. We then advise on the strategies to mitigate or reduce those risks to protect corporate reputation and public health.
Click to download
Developing and commercializing new inhaled drug products, requires huge energy and deep scientific understanding. This level of resource and subject matter expertise can be difficult to maintain inside your business, and that is where we can help.
Click to download
Learn more about our UK and EU Novel Food Application scientific consultancy and analytical testing support.
Click to download
Our product development and regulatory experts can help you meet expectations at key milestones in your product development project to better inform decision making and investment choices.
Click to download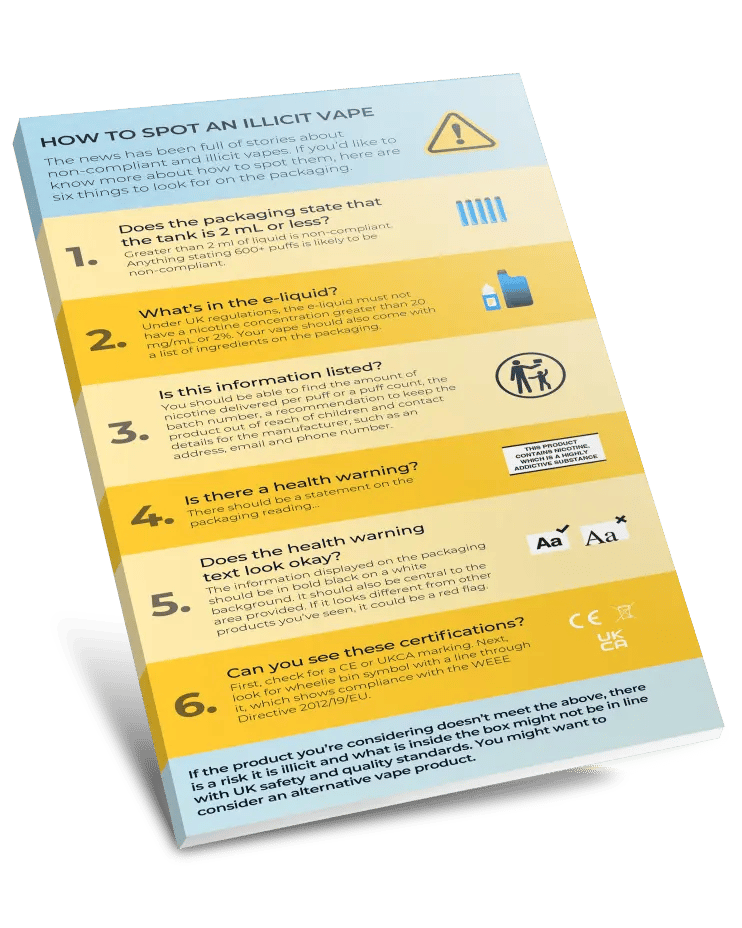
Learn about six easy-to-spot packaging violations that could indicate a vape is illicit and shouldn't be on sale in the UK and EU.
Click to download
The rapid growth of the cannabis market has outpaced the establishment of comprehensive safety standards and regulations. To help create a pathway towards safe, secure, and reliably-regulated CBD products, Cristelle Santos, Consultant Toxicologist at Broughton, has proposed a safety framework.
Click to download
We believe that the sectors we work within expect a continually improving service where we constantly strive to improve processes and Quality.
Click to download
Explore Paul Hardman's latest research comparing HPLC-UV and GC-FID methods for nicotine quantification presented at CORESTA PSPT 2025. Learn how method variability impacts product consistency, regulatory compliance, and cost-effective quality control across nicotine products.
Click to download
ToxHQ by Broughton offers automated, data-driven risk assessments and delivers fast, high-quality toxicological evaluations, so you can launch safer products with confidence.
Click to download
Explore HTP Regulations and Testing in our scientific poster presented at CORESTA's Annual Congress and the Tobacco Science Research Conference
Click to download
In this whitepaper, we discuss the stability studies that may be performed during pharmaceutical development and in support of a regulatory dossier to support the licensing of a medicinal drug product.
Click to download
The US FDA has granted Marketing Orders for four menthol-flavoured pe-cigarette products. This marks the first time that the FDA has granted MOs for non-tobacco flavoured products via the PMTA pathway.
Click to download
Product Lifecycle Manager, Malcolm Saxton was featured in the June edition of NGP Trends discussing the most effective way to design a heated tobacco product.
Click to download
In this whitepaper, we discuss the stability studies that may be performed during pharmaceutical development and in support of a regulatory dossier to support the licensing of a medicinal drug product.
Click to download
Extractables and leachables (E&L) risk assessments are valuable processes that can identify and highlight the risks of potential leachables from both the container closure system and the manufacturing processes. The risk assessments also include the level of risk that leachables might present to user safety and product quality. Download this Whitepaper to learn more.
Click to download
There is no doubt that the reduced-risk industry today is at an impasse, although there is now common acceptance that combustible cigarettes are the most hazardous form of nicotine delivery due to the accompanying harmful chemicals produced from tobacco combustion.
Click to download.png?width=2381&height=2977&name=UK%20Disposables%20Vape%20Ban%20(Thumbnail).png)
Rishi Sunak recently announced that the UK Government would ban disposable vapes as part of its plan to tackle the rise in youth vaping. Paul Hardman shares his thoughts on the announcement and what may come next.
Click to download
In this whitepaper, we discuss the range of validated analytical methods required to support the comprehensive characterization of various dosage forms of cannabinoid products.
Click to download
Since their introduction in 1956, pressurised metered dose inhalers (pMDIs) have become the dominant treatment choice for patients suffering from common respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). However, unbeknown to most patients and many doctors, pMDIs account for 3.9% of NHS’s annual carbon emissions. 1Here we will discuss how formulation changes might be the answer to the pMDI-related sustainability problem.
Click to download
Both the FDA and EFSA have raised several concerns about CBD as supplements and as an ingredient in food and drinks, citing potential liver damage as a possible side effect. Is CBD really that toxic to the liver? To find out, toxicologist Cristelle Santos looks at the scientific evidence.
Click to download
Broughton share their thoughts on how to reduce costs and protect timelines while continuing to innovate.
Click to download
Broughton summarises the regulatory pathways available for cannabinoid products in the UK market and the legal requirements for each potential market route.
Click to download
To develop cannabis products that can both enhance the quality of life of cannabis users and comply with current and future regulations, we believe that fi rst we need to understand what cannabis is, how people use it, and how it affects the human body and mind. This white paper aims to provide essential but concise information about these areas, supported by scientific evidence.
Click to download
In this whitepaper, Broughton discusses the susceptibility of cannabinoids to degrade and summarizes the main degradation pathways of the primary cannabinoid products on the market; tetrahydrocannabinol (THC) and Cannabidiol (CBD).
Click to download
Our Senior Consultant, Malcolm Saxton presented a poster at the 76th Tobacco Science Research Conference in Norfolk, Virginia USA about ‘E-Cigarette Regulatory Non-Compliance in the UK Marketplace’.
Click to download
The adaptation of e-cigarettes as drug delivery devices holds great potential for inhalation therapy. So, can we apply the advances made in nicotine delivery to other active substances?
Click to download
3 minute read
Last week’s announcement from FDA regarding the authorization of on! PLUS was very encouraging.
3 minute read
For much of the past decade, the US nicotine and ENDS marketplace has operated in a state of regulatory imbalance.
5 minute read
~ Integrating testing can help satisfy regulators ~.
Helping you and your business stay ahead of the competition with regular updates on the latest regulatory changes and industry news.